Denial and retirement savings insanity
The career of the Compliance Officer By Greta Maritz 1. Introduction he concept of a formalised compliance function is relatively new in SouthAfrica compared to global markets. Historically, the only organisations thathad semblances of compliance officers or functions, were those who wererequired to have them in terms of exchange rules. However, since the late‘90s, the concept ha
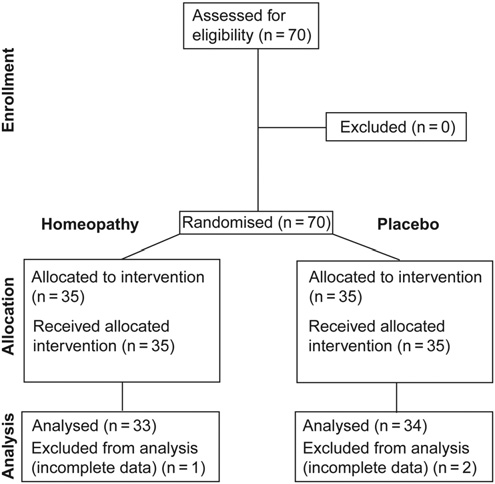 Homeopathic medicine has been used for about two centuries. Several studies describe its superiority above placebo.
Homeopathic medicine has been used for about two centuries. Several studies describe its superiority above placebo.