Microsoft word - newsletter nov 2005.mht
Janusz Korczak International Newsletter no 16 (16 pages) Amsterdam December 2005. Dear friends and colleagues. The last Korczak Newsletter of 2005. Overlooking all the information in the preceding LETTERS the conclusion must be: a fruitful year. Many activities in the Korczak Associations, conferences, publications, seminars etc. We also noticed that the Korczak Newsletter brought people

 Journal of Organometallic Chemistry 558 (1998) 41 – 49
Synthesis and structure of methylpalladium(II) and -platinum(II)
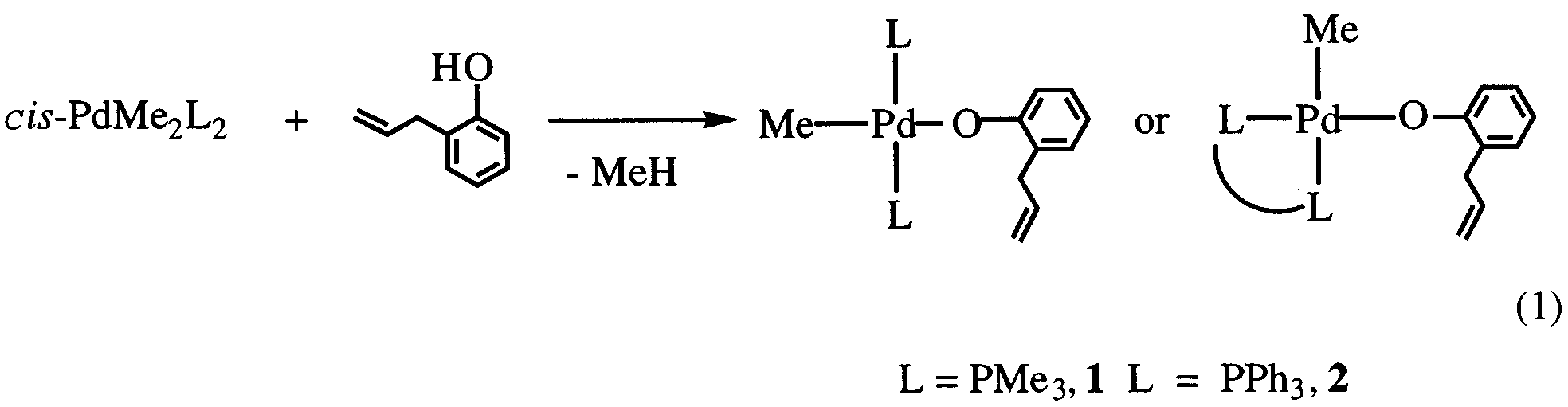
trans-PdMe(O H CH CH CH -o)(PR ) (R
p2-C,C-OC H CH CH CH -o)(PMe ). Simple O-coordination and
chelating coordination depending on the metal center and auxiliary
Yong-Joo Kim a,*, Jae-Young Lee a, Kohtaro Osakada b
a Department of Chemistry, Kangnung National Uni6ersity, Kangnung, 210-702, South Korea
b Research Laboratory of Resources Utilization, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226, Japan
Received 8 July 1997; received in revised form 2 December 1997
Abstract
Journal of Organometallic Chemistry 558 (1998) 41 – 49
Synthesis and structure of methylpalladium(II) and -platinum(II)
trans-PdMe(O H CH CH CH -o)(PR ) (R
p2-C,C-OC H CH CH CH -o)(PMe ). Simple O-coordination and
chelating coordination depending on the metal center and auxiliary
Yong-Joo Kim a,*, Jae-Young Lee a, Kohtaro Osakada b
a Department of Chemistry, Kangnung National Uni6ersity, Kangnung, 210-702, South Korea
b Research Laboratory of Resources Utilization, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226, Japan
Received 8 July 1997; received in revised form 2 December 1997
Abstract
 Y.-J. Kim et al. / Journal of Organometallic Chemistry 558 (1998) 41 – 49
(II), -palladium (II), and -platinum (II) phenoxides or
caused by coupling with two unequivalent phosphorus
complexes, MR(OAr)L · (HOAr) containing phenol
nuclei. The 1H- and 13C-NMR signals of PMe ligands
associated with phenoxido ligands through hydrogen
of 1 and 13C{1H}-NMR peak due to ipso carbons of
Y.-J. Kim et al. / Journal of Organometallic Chemistry 558 (1998) 41 – 49
(II), -palladium (II), and -platinum (II) phenoxides or
caused by coupling with two unequivalent phosphorus
complexes, MR(OAr)L · (HOAr) containing phenol
nuclei. The 1H- and 13C-NMR signals of PMe ligands
associated with phenoxido ligands through hydrogen
of 1 and 13C{1H}-NMR peak due to ipso carbons of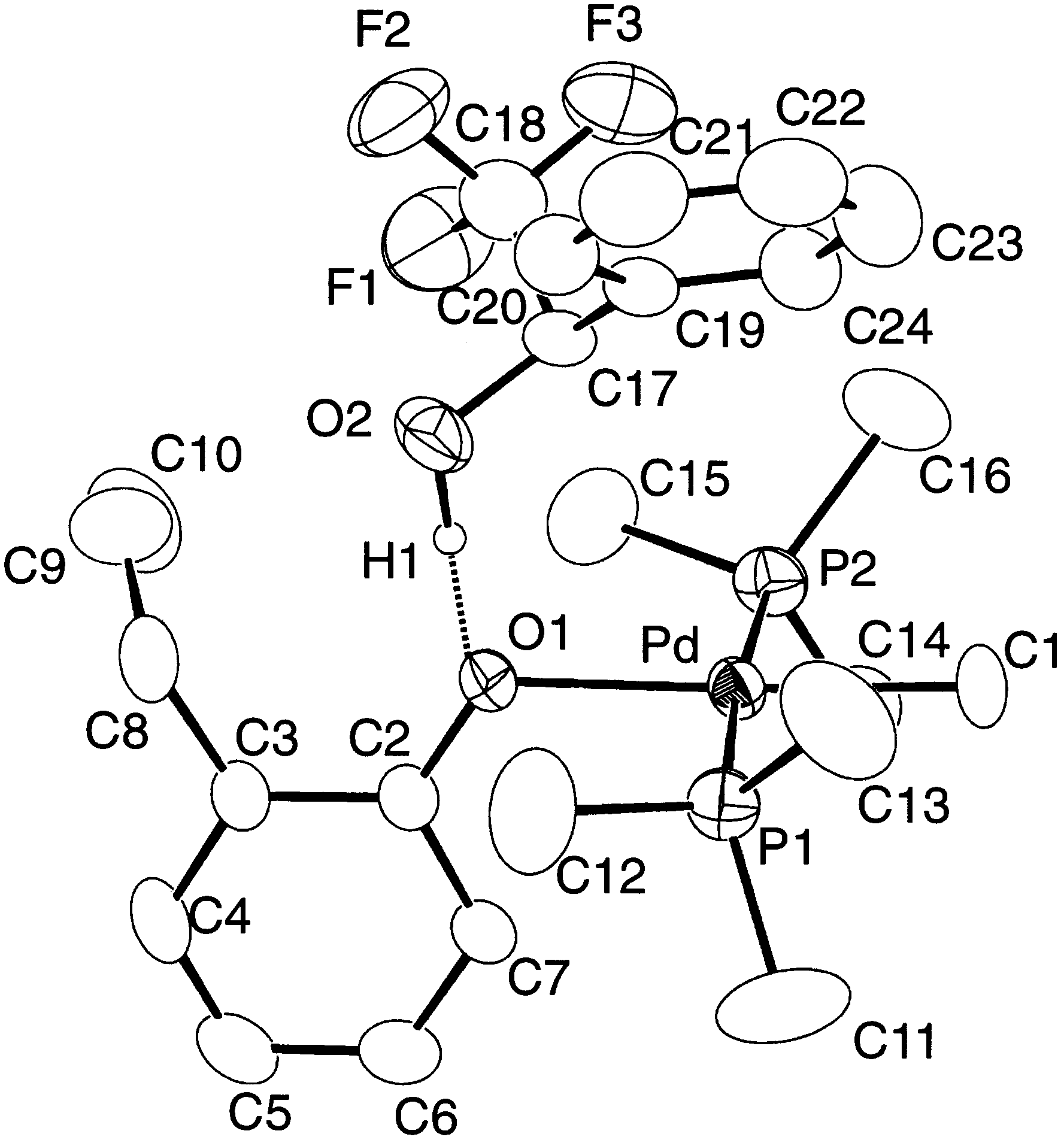
 Y.-J. Kim et al. / Journal of Organometallic Chemistry 558 (1998) 41 – 49
The 1H-NMR spectrum of 4 taken in CDCl (25°C)
Y.-J. Kim et al. / Journal of Organometallic Chemistry 558 (1998) 41 – 49
The 1H-NMR spectrum of 4 taken in CDCl (25°C)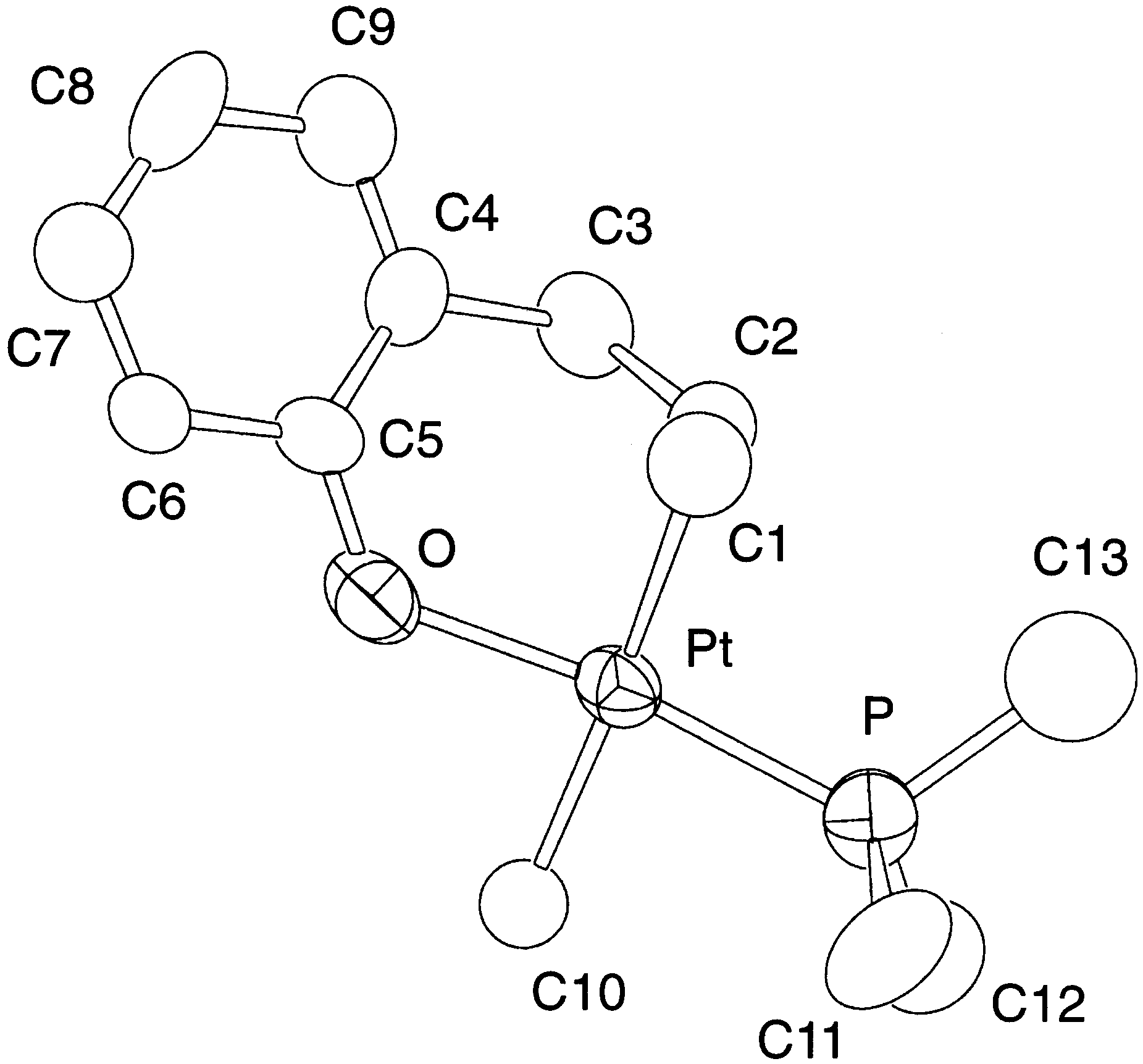
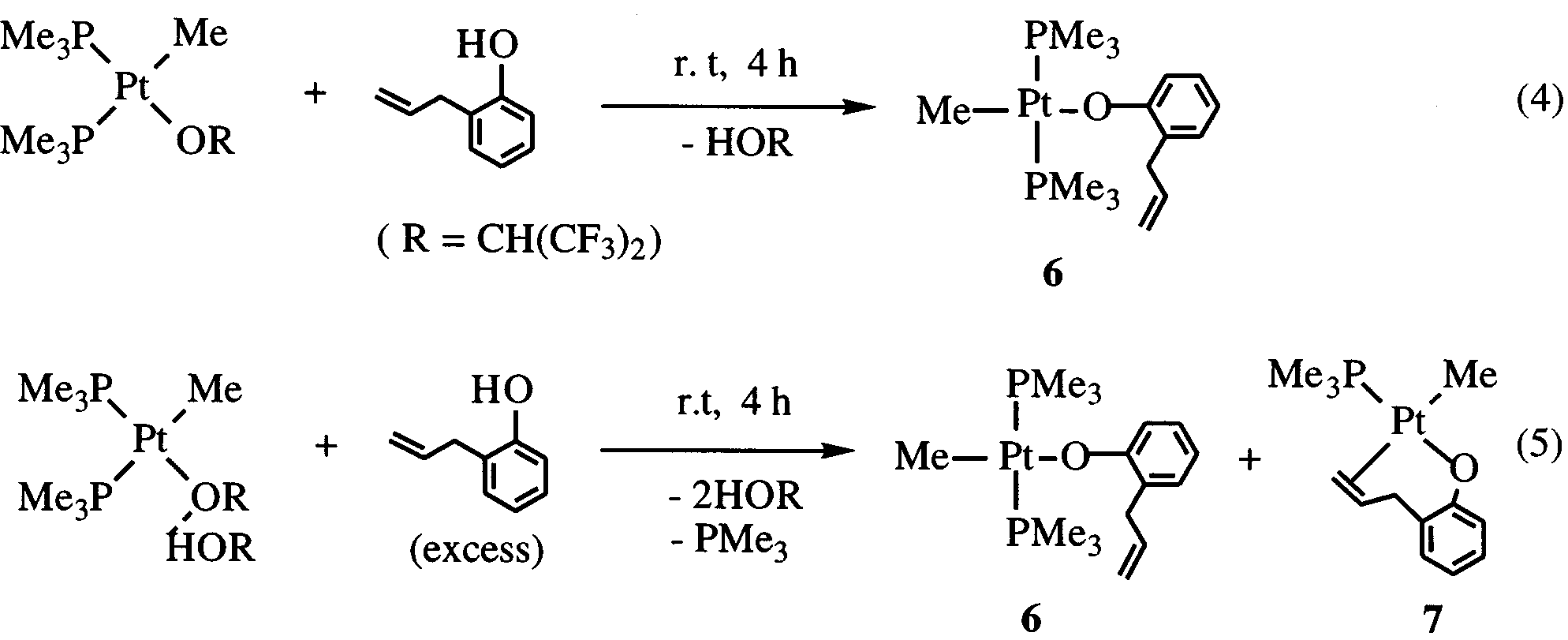 Y.-J. Kim et al. / Journal of Organometallic Chemistry 558 (1998) 41 – 49
CH -o)(PMe ) (7) which are isolated by fractional
Y.-J. Kim et al. / Journal of Organometallic Chemistry 558 (1998) 41 – 49
CH -o)(PMe ) (7) which are isolated by fractional